Hydrogen bond: A fascination forever! |
E. ArunanIn 1920, Latimer and Rodebush conceptualized the hydrogen bond (H bond)1. Almost eight decades have passed and the H bond is continuing to fascinate researchers in the fields of physics, chemistry, biology and materials sciences. Literally, thousands of papers are published every year on H bonds (A Chemical Abstract search for the year 1998 with ‘hydrogen bond’ as the key word resulted in 3707 documents and 13436 occurrences!). Some of the recent publications deserve a closer look as they address some fundamental issues on H bonds.
Hydrogen bond has been generally thought of as originating from electrostatic interactions such as interaction between two dipoles. Electron sharing or covalent nature was considered not to be important. This view was strengthened by the fact that electrostatic models, such as the one by Buckingham and Fowler, were able to explain/predict the H bond geometry fairly accurately2. However, in 1949 Pauling3 had empirically calculated that H bond would have 5% covalent character in an O––H-----O bond. It has taken almost 50 years for experimentalists to verify Pauling’s empirical estimate. Two techniques, NMR and Compton scattering, have recently given convincing evidence that Pauling was right! The NMR experiment can, in principle, give some estimate of covalent nature if one could see the spin–spin coupling between the two other nuclei involved in the H bond. It has been done now.
Dingley and Grzesiek4 used a novel pulse sequence in their NMR experiment to observe J coupling between two 15N nuclei (2JNN) connected through an H bond, N–H-----N, in nucleic acid–base pairs. Spin–spin or scalar coupling, also known as J coupling, is generally observed between nuclei that are connected by covalent bonds. The magnitude of the J coupling depends on the extent of the orbital overlap in a bond. It was widely believed that the covalent nature of the H bonds would be too insignificant and it may at best produce a small splitting of << 1 Hz due to J coupling5. Dingley and Grzesiek observed a splitting of 7 Hz! This was the first time J coupling could be observed between two nuclei through an H bond, which implies that the H bond has some covalent character (Figure 1). The key to their experimental success was the fact that they were using a uniformly 13C/15N enriched sample. Since this observation, several groups have found similar and higher J coupling between nuclei connected through an H bond. Golubev et al.6 have observed a splitting of 96 Hz between 19F and 15N in an
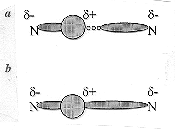
Figure 1. Schematic representations of the hydrogen bond. a, The s bond is depicted as the overlap of the hydrogen s-orbital (represented by the circle) and a nitrogen sp3 orbital. The H bond is depicted as the interaction between the positive end of the dipole in N–H and the negative end of the other N. b, The lone pair electrons are shown spending a non-negligible amount of time in the vicinity of the hydrogen, though the electrostatic interaction is still present and dominant. Though it is a small effect, it is significant as it leads to an essentially continuous wave function between the two N atoms. This causes the spin–spin coupling in the NMR experiment and the two additional peaks in the Compton scattering experiment. (Figure adapted from ref. 9 with permission.)
F–H-----N bond of an acid–base complex. Grzesiek’s group7 has observed a coupling of 15 Hz through 3 bonds, 3JNC, in proteins with N–H-----O–C hydrogen bond. These observations have not yet been utilized to determine the extent of covalent character in an H bond. However, such an estimate has been made with Compton scattering data in common ice.
A Compton scattering experiment by US, French and Canadian physicists, utilizing the European Synchrotron Radiation Facility in Grenoble has been recently reported8. Just as Rayleigh scattering of UV-visible radiation, we have Thomson scattering of X-rays which forms the basis of standard X-ray crystallography. Compton scattering is an X-ray analog of the Raman scattering, which is more familiar to chemists (To be fair, Raman discovered the optical analog of Compton scattering for which he won the Nobel Prize two years after Compton did). Compton scattering leads to the momentum space profile of the wave functions of the valence electrons in a molecule. One can Fourier transform this to find how the electron density is distributed in the molecule. In ice, the authors found three peaks at 0.89, 1.72 and 2.85 ┼. These distances give extents to which wave functions of the bonding electrons are distributed. For example, the peak at 0.89 ┼ corresponds to the O–H bond and indicates that wave functions of the electrons involved in this bond extend over this distance. The 2.85 ┼ peak corresponds to the nearest neighbour O–O distance and 1.75 ┼ peak corresponds to H bond distance. These two peaks are new and significant, as they indicate that there are bonding wave functions extending in space between O–O, with part of it in the H bond, as in Figure 1. This clearly demonstrates that there is electron sharing in the H bond. The results indicate ╗ 10% covalent character in the O––H----O hydrogen bond. This is in remarkable agreement with Pauling’s estimate, considering the fact that Pauling used empirical relations based on intuition. This estimate is as yet unpublished but reported in a news article in Nature: Structural Biology9.
Recently, Hobza and coworkers from Heyrovsky Institute of Physical Chemistry, Czech Republic, claim to have observed what they call an anti H bond10. In general, H bond formation leads to lengthening of the donor X––H bond and a concomitant red shift in the X––H stretching frequency. However, for two complexes namely flouroform-ethylene oxide11 and chloroform-fluoro-benzene12, the C–H bond which is involved in the H bond appears to show a blue shift and bond contraction! The authors have been successful in using ab initio calculations that support their experimental observations. They conclude that dispersion forces play a major role in these interactions. In a normal H bond, the bond lengthening in the donor leads to enhancement of dipole moment and more stability in the dipole–dipole interaction. Dispersion energy is proportional to the higher power of reciprocal distance between the centers of mass of both sub systems. To minimize the distance and maximize the attraction, it is advantageous to contract the C–H bond of the proton donor. This contraction, they claim, can lead to shorter intermolecular distances and larger dispersion energy. According to these workers, the anti-H bond is not limited to the gas phase complexes. The C–H----O contact in adenine–thymine base pair also exhibits such character13. The T-shaped benzene dimer too has anti H bond! This observation, if true, is very important as the benzene dimer is the simplest model for aromatic–aromatic interactions in biological environments.
Ever since the H bond was conceptualized, chemists (knowing their love for Periodic Table) must have wondered about the possibilities of other atoms exhibiting similar interactions. Two recent papers in the Angewandte Chemie appear to give convincing evidence for halogen bonds! Resnati and coworkers14 have reported single crystal X-ray studies on (S)-1,2-dibromohexafluoro-propane which was crystallized in 100% enantiomeric excess from a solution of racemate with (–) sparteine hydrobromide. They suggest that the interaction between the compounds through a halogen bond, analogous to an H bond, facilitates the preferential crystallization of one form. More importantly, Legon of University of Exeter has reviewed15 rotational spectroscopic studies on a series of HX and XY (X and Y –halogens) complexes with Lewis bases, B. These complexes were formed in gas
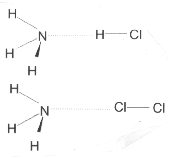
Figure 2. Experimentally observed structures (C3v, not to scale) for H3N---HCl and H3N---Cl2. The intermolecular stretching force constant for HCl complex is 17.6 N m–1 and that for Cl2 complex is 12.7 N m–1. In the gas phase H3N---HCl exists as an H bonded complex and not as ammonium chloride. The N---H distance is about 1.9 ┼ compared to the N–H distance of 1 ┼ in ammonia. The distance between N and the bonded chlorine is 2.7 ┼ in NH3–Cl2 complex, which is about 0.6 ┼ shorter than the sum of van der Waals radii for N and Cl atoms. Typical N–Cl bond distance, for example in NCl3, is about 1.7 ┼. The H–Cl bond forms an angle of 13.4░ with respect to the intermolecular bond while the Cl–Cl bond is at 7.5░ from the intermolecular bond. Halogen bonds are in general more collinear than the H bond. (Figure adapted from ref. 15 with permission.)
phase by supersonic expansion and observed by Fourier transform microwave spectrometer. Thus they are direct observation of the halogen bond. Legon’s group has done systematic studies on these complexes with B = CO, C2H2, C2H4, HCN, H2S, NH3, pyridine, furan, thiophen, cyclopropane, methylene cyclopropane, etc. and HX/XY = HF, HCl, HBr, HI, F3, Cl2, Br2, BrCl, ClF and ICl. The B---HX and B---XY complexes were found to be isostructural in all the cases. Figure 2 shows the geometry of NH3---HCl and NH3---Cl2 complexes, both having C3v point group. Ironically, it has been found that the B---XY complexes are more collinear than the H bonded B---HX. For example, in thiirane–ClF complex, the deviation from linearity is found to be 3.5 ▒ 2░ while that in thiirane–HCl complex is found to be 21 ▒ 5░ . Also, in several cases the B---XY bond has been found to be stronger than B---HY bond. This extensive study clearly demonstrates the presence of a halogen bond.
Pimentel and McLellan wrote the first definitive book on hydrogen bonds16. They have given a very practical definition of the hydrogen bond which follows: A hydrogen bond is said to exist when 1) there is evidence of a bond and 2) there is evidence that this bond specifically involves a hydrogen atom already bonded to another atom. According to this definition, we see that both the H bond and the anti-H bond can be classified as just H bond! Also, it justifies the halogen bond description discussed above. What would be the extent of covalent character in H bonds observed in various surroundings? What would be the nature of the halogen bond? It is clear that in the next few decades, we will continue to see growing literature on the H bond or intermolecular interactions, in general!
Note added in proof: Very recently, Scheurer and BrŘschweiler17 have reported quantum chemical studies, using density functional theory (DFT), on the spin–spin couplings across H bonds. They conclude that the Fermi contact terms, originating from orbitals with s bond character across the H bond, make the major contributions to the spin–spin couplings experimentally observed.
- Latimer, W. M. and Rodebush, W. H., J. Am. Chem. Soc., 1920, 42, 1419.
- Buckingham, A. D. and Fowler, P. W., J. Chem. Phys., 1983, 79, 6426.
- Pauling, L., The Nature of the Chemical Bond, Cornell University Press, Ithaca, NY, 1960, p. 453.
- Dingley, A. J. and Grzesiek, S., J. Am. Chem. Soc., 1998, 120, 8293.
- Cornilescu, G., Hu, J-S. and Bax, A.,
J. Am. Chem. Soc., 1999, 121, 2949; Borman, S., Chem. Eng. News, 10 May 1999, p. 36.- Golubev, N. S. et al., Chem. Eur. J., 1999, 5, 492.
- Cordier, F. and Grzesiek, S., J. Am. Chem. Soc., 1999, 121, 1601.
- Isaacs, E. D. et al., Phys. Rev. Lett., 1999, 82, 600.
- Martin, T. W. and Derewenda, Z. S., Nature Struct. Biol., 1999, 6, 403.
- Cubero E. et al., J. Phys. Chem., 1999, A103, 6394.
- Hobza, P. and Havlas, Z., Chem. Phys. Lett., 1999, 303, 447.
- Hobza, P. et al., Chem. Phys. Lett., 1999, 293, 180.
- Hobza, P. et al., J. Phys. Chem., 1998, A102, 2501.
- Resnati, G. et al., Angew. Chem. Int. Ed. Engl., 1999, 38, 2433.
- Legon, A. C., Angew. Chem. Int. Ed. Engl., 1999, 38, 2686.
- Pimentel, G. C. and McLellan, A. L., The Hydrogen Bond, W. H. Freeman and Co., San Francisco, 1960.
- Scheurer, C. and BrŘschweiler, R.,
J. Am. Chem. Soc., 1999, 121, 8661.ACKNOWLEDGEMENTS. I thank Profs.
K. L. Sebastian and A. G. Samuelson for stimulating discussions. I thank Prof.
P. Balaram for bringing refs 9 and 17 to my attention.E. Arunan is in the Inorganic and Physical Chemistry Department, Indian Institute of Science, Bangalore 560 012, India